Cancer mRNA vaccines: clinical advances and future opportunities
mRNA vaccines have been revolutionary in terms of their rapid development and prevention of SARS-CoV-2 infections during the COVID-19 pandemic, and this technology has considerable potential for application to the treatment of cancer. Compared with traditional cancer vaccines based on proteins or peptides, mRNA vaccines reconcile the needs for both personalization and commercialization in a manner that is unique to each patient but not beholden to their HLA haplotype. A further advantage of mRNA vaccines is the availability of engineering strategies to improve their stability while retaining immunogenicity, enabling the induction of complementary innate and adaptive immune responses. Thus far, no mRNA-based cancer vaccines have received regulatory approval, although several phase I–II trials have yielded promising results, including in historically poorly immunogenic tumours. Furthermore, many early phase trials testing a wide range of vaccine designs are currently ongoing. In this Review, we describe the advantages of cancer mRNA vaccines and advances in clinical trials using both cell-based and nanoparticle-based delivery methods, with discussions of future combinations and iterations that might optimize the activity of these agents.
Key points
- The advent of mRNA-based vaccines against SARS-CoV-2 has ushered in a new era of mRNA vaccines against other infectious diseases as well as cancer.
- Advantages of directly injected mRNA vaccines include safety and flexibility in terms of the speed with which personalized epitopes or antigens can be produced in the form of mRNA.
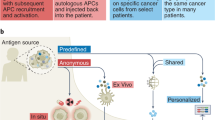
- mRNA is a labile molecule and is best delivered in nanoparticles, which are not currently developed to target specific cell types for the induction of immune responses. Alternatively, mRNA can be loaded into antigen-presenting cells that can then be administered as a vaccine.
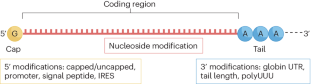
- SARS-CoV-2 vaccines use mRNA incorporating modified nucleotides to minimize the risk of a type I interferon response. However, this response might be beneficial in the setting of cancer vaccines, which must generate immune responses against antigens that are overexpressed self-antigens or those that result in only slightly altered proteins and are therefore likely to be less immunogenic.
- The future of mRNA vaccines will include optimization of the nanoparticles used to deliver the vaccine as well as of the mRNA itself.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Therapeutic cancer vaccines
Article 27 April 2021
Cancer vaccines: the next immunotherapy frontier
Article 23 August 2022
Incorporation of a Toll-like receptor 2/6 agonist potentiates mRNA vaccines against cancer and infectious diseases
Article Open access 17 July 2023
References
- McCarthy, E. F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J.26, 154–158 (2006). PubMedPubMed CentralGoogle Scholar
- van der Burg, S. H., Arens, R., Ossendorp, F., van Hall, T. & Melief, C. J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer16, 219–233 (2016). ArticlePubMedGoogle Scholar
- Pollack, I. F. et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J. Clin. Oncol.32, 2050–2058 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sampson, J. H. et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol.28, 4722–4729 (2010). ArticlePubMedPubMed CentralGoogle Scholar
- Prins, R. M., Cloughesy, T. F. & Liau, L. M. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N. Engl. J. Med.359, 539–541 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Shi, G. N. et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials113, 191–202 (2017). ArticleCASPubMedGoogle Scholar
- Duraiswamy, J., Kaluza, K. M., Freeman, G. J. & Coukos, G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res.73, 3591–3603 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Coban, C. et al. Novel strategies to improve DNA vaccine immunogenicity. Curr. Gene Ther.11, 479–484 (2011). ArticleCASPubMedGoogle Scholar
- Lancet Commission on COVID-19 Vaccines and Therapeutics Task Force Members.Operation warp speed: implications for global vaccine security. Lancet Glob. Health9, e1017–e1021 (2021). ArticleGoogle Scholar
- U.S. Department of Health and Human Services. Explaining Operation Warp Speedhttps://www.nihb.org/covid-19/wp-content/uploads/2020/08/Fact-sheet-operation-warp-speed.pdf (accessed May 2024).
- Mikulic, M. Number of COVID-19 vaccine doses administered in the United States as of April 26, 2023, by vaccine manufacturer. statista, https://www.statista.com/statistics/1198516/covid-19-vaccinations-administered-us-by-company/ (accessed May 2024).
- Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature618, 144–150 (2023). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yang, A., Farmer, E., Wu, T. C. & Hung, C. F. Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci.23, 75 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Lei, J. et al. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med.383, 1340–1348 (2020). ArticleCASPubMedGoogle Scholar
- Kantarjian, H. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med.376, 836–847 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Navid, F. et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J. Clin. Oncol.32, 1445–1452 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med.363, 1324–1334 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med.371, 1507–1517 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med.363, 711–723 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Riedmann, E. M. World’s first cancer vaccine licensed: PROVENGE ® . Hum. Vaccin.6, 430–435 (2010). Google Scholar
- Bot, A. The landmark approval of Provenge, what it means to immunology and “in this issue”: the complex relation between vaccines and autoimmunity. Int. Rev. Immunol.29, 235–238 (2010). ArticleCASPubMedGoogle Scholar
- Brower, V. Approval of provenge seen as first step for cancer treatment vaccines. J. Natl Cancer Inst.102, 1108–1110 (2010). ArticlePubMedGoogle Scholar
- Jahnisch, H. et al. Dendritic cell-based immunotherapy for prostate cancer. Clin. Dev. Immunol.2010, 517493 (2010). PubMedPubMed CentralGoogle Scholar
- Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med.363, 411–422 (2010). ArticleCASPubMedGoogle Scholar
- Paller, C. J. & Antonarakis, E. S. Sipuleucel-T for the treatment of metastatic prostate cancer: promise and challenges. Hum. Vaccin. Immunother.8, 509–519 (2012). ArticleCASPubMedGoogle Scholar
- Sheikh, N. A. et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol. Immunother.62, 137–147 (2013). ArticleCASPubMedGoogle Scholar
- Sanchez-Perez, L. et al. Potent selection of antigen loss variants of B16 melanoma following inflammatory killing of melanocytes in vivo. Cancer Res.65, 2009–2017 (2005). ArticleCASPubMedGoogle Scholar
- Bloch, O. et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res.19, 3165–3175 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Morse, M. A. et al. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res.59, 56–58 (1999). CASPubMedGoogle Scholar
- Quillien, V. et al. Biodistribution of radiolabelled human dendritic cells injected by various routes. Eur. J. Nucl. Med. Mol. Imaging32, 731–741 (2005). ArticleCASPubMedGoogle Scholar
- Bandola-Simon, J. & Roche, P. A. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol. Immunol.113, 31–37 (2019). ArticleCASPubMedGoogle Scholar
- Demoulin, S., Herfs, M., Delvenne, P. & Hubert, P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J. Leukoc. Biol.93, 343–352 (2013). ArticleCASPubMedGoogle Scholar
- Oble, D. A., Loewe, R., Yu, P. & Mihm, M. C. Jr Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun.9, 3 (2009). PubMedPubMed CentralGoogle Scholar
- Hutchison, S. et al. Characterization of myeloid-derived suppressor cells and cytokines GM-CSF, IL-10 and MCP-1 in dogs with malignant melanoma receiving a GD3-based immunotherapy. Vet. Immunol. Immunopathol.216, 109912 (2019). ArticleCASPubMedGoogle Scholar
- Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med.366, 2455–2465 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Lohr, J. et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin. Cancer Res.17, 4296–4308 (2011). ArticleCASPubMedGoogle Scholar
- Hwang, S. L., Chung, N. P., Chan, J. K. & Lin, C. L. Indoleamine 2, 3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res.15, 167–175 (2005). ArticleCASPubMedGoogle Scholar
- Toda, Y. et al. PD-L1 and IDO1 expression and tumor-infiltrating lymphocytes in osteosarcoma patients: comparative study of primary and metastatic lesions. J. Cancer Res. Clin. Oncol.146, 2607–2620 (2020). ArticleCASPubMedGoogle Scholar
- Wainwright, D. A. et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res.18, 6110–6121 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Tavernier, G. et al. mRNA as gene therapeutic: how to control protein expression. J. Control. Rel.150, 238–247 (2011). ArticleCASGoogle Scholar
- Malone, R. W., Felgner, P. L. & Verma, I. M. Cationic liposome-mediated RNA transfection. Proc. Natl Acad. Sci. USA86, 6077–6081 (1989). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science247, 1465–1468 (1990). ArticleCASPubMedGoogle Scholar
- Martinon, F. et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol.23, 1719–1722 (1993). ArticleCASPubMedGoogle Scholar
- Conry, R. M. et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res.55, 1397–1400 (1995). CASPubMedGoogle Scholar
- Johanning, F. W. et al. A Sindbis virus mRNA polynucleotide vector achieves prolonged and high level heterologous gene expression in vivo. Nucleic Acids Res.23, 1495–1501 (1995). ArticleCASPubMedPubMed CentralGoogle Scholar
- Aliahmad, P., Miyake-Stoner, S. J., Geall, A. J. & Wang, N. S. Next generation self-replicating RNA vectors for vaccines and immunotherapies. Cancer Gene Ther.30, 785–793 (2023). ArticleCASPubMedGoogle Scholar
- Morse, M. A. et al. Clinical trials of self-replicating RNA-based cancer vaccines. Cancer Gene Ther.30, 803–811 (2023). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ulmer, J. B., Mason, P. W., Geall, A. & Mandl, C. W. RNA-based vaccines. Vaccine30, 4414–4418 (2012). ArticleCASPubMedGoogle Scholar
- Schlake, T., Thess, A., Fotin-Mleczek, M. & Kallen, K. J. Developing mRNA-vaccine technologies. RNA Biol.9, 1319–1330 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Boczkowski, D., Nair, S. K., Nam, J. H., Lyerly, H. K. & Gilboa, E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res.60, 1028–1034 (2000). CASPubMedGoogle Scholar
- Boczkowski, D., Nair, S. K., Snyder, D. & Gilboa, E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med.184, 465–472 (1996). ArticleCASPubMedGoogle Scholar
- Nair, S. K. et al. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat. Biotechnol.16, 364–369 (1998). ArticleCASPubMedGoogle Scholar
- Heiser, A. et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J. Clin. Invest.109, 409–417 (2002). ArticleCASPubMedPubMed CentralGoogle Scholar
- Van Tendeloo, V. F. et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood98, 49–56 (2001). ArticlePubMedGoogle Scholar
- Zhang, S. N. et al. Optimizing DC vaccination by combination with oncolytic adenovirus coexpressing IL-12 and GM-CSF. Mol. Ther.19, 1558–1568 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Khoury, H. J. et al. Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia. Cancer123, 3061–3072 (2017). ArticleCASPubMedGoogle Scholar
- Anguille, S. et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood130, 1713–1721 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Boudewijns, S. et al. Autologous monocyte-derived DC vaccination combined with cisplatin in stage III and IV melanoma patients: a prospective, randomized phase 2 trial. Cancer Immunol. Immun.69, 477–488 (2020). ArticleCASGoogle Scholar
- Kongsted, P. et al. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: a randomized phase II study. Cytotherapy19, 500–513 (2017). ArticleCASPubMedGoogle Scholar
- Batich, K. A., Mitchell, D. A., Healy, P., Herndon, J. E. & Sampson, J. H. Once, twice, three times a finding: reproducibility of dendritic cell vaccine trials targeting cytomegalovirus in glioblastoma. Clin. Cancer Res.26, 5297–5303 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
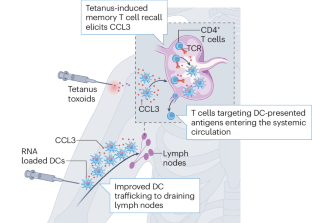
- Mitchell, D. A. et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature519, 366–369 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Batich, K. A. et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin. Cancer Res.23, 1898–1909 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Batich, K. A., Swartz, A. M. & Sampson, J. H. Preconditioning vaccine sites for mRNA-transfected dendritic cell therapy and antitumor efficacy. Methods Mol. Biol.1403, 819–838 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Wilgenhof, S. et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J. Clin. Oncol.34, 1330 (2016). ArticlePubMedGoogle Scholar
- Dannull, J. et al. Melanoma immunotherapy using mature DCs expressing the constitutive proteasome. J. Clin. Invest.123, 3135–3145 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dannull, J. et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest.115, 3623–3633 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Su, Z. et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res.63, 2127–2133 (2003). CASPubMedGoogle Scholar
- Heiser, A. et al. Human dendritic cells transfected with renal tumor RNA stimulate polyclonal T-cell responses against antigens expressed by primary and metastatic tumors. Cancer Res.61, 3388–3393 (2001). CASPubMedGoogle Scholar
- Caruso, D. A. et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol.6, 236–246 (2004). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sayour, E. J. et al. Personalized tumor RNA loaded lipid-nanoparticles prime the systemic and intratumoral milieu for response to cancer immunotherapy. Nano Lett.18, 6195–6206 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Nair, S. K. et al. Ex vivo generation of dendritic cells from cryopreserved, post-induction chemotherapy, mobilized leukapheresis from pediatric patients with medulloblastoma. J. Neurooncol.125, 65–74 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Figlin, R. A. et al. Results of the ADAPT phase 3 study of rocapuldencel-T in combination with sunitinib as first-line therapy in patients with metastatic renal cell carcinoma. Clin. Cancer Res.26, 2327–2336 (2020). ArticleCASPubMedGoogle Scholar
- DeBenedette, M., Gamble, A., Norris, M., Horvatinovich, J. & Nicolette, C. A. A review of the clinical experience with CMN-001, a tumor RNA loaded dendritic cell immunotherapy for the treatment of metastatic renal cell carcinoma. Hum. Vaccin. Immunother.19, 2220629 (2023). ArticlePubMedPubMed CentralGoogle Scholar
- Lorentzen, C. L., Haanen, J. B., Met, O. & Svane, I. M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol.23, e450–e458 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Benteyn, D., Heirman, C., Bonehill, A., Thielemans, K. & Breckpot, K. mRNA-based dendritic cell vaccines. Expert Rev. Vaccin.14, 161–176 (2015). ArticleCASGoogle Scholar
- Nair, S., Boczkowski, D., Pruitt, S. & Urban, J. in Cancer Vaccines: From Research to Clinical Practice 1st ed (Eds Bot, A., Obrocea, M. & Marincola, F. M.) 217–231 (2011).
- Dorrie, J., Schaft, N., Schuler, G. & Schuler-Thurner, B. Therapeutic cancer vaccination with ex vivo RNA-transfected dendritic cells-an update. Pharmaceutics12, 92 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Van Driessche, A. et al. Clinical-grade manufacturing of autologous mature mRNA-electroporated dendritic cells and safety testing in acute myeloid leukemia patients in a phase I dose-escalation clinical trial. Cytotherapy11, 653–668 (2009). ArticleCASPubMedGoogle Scholar
- Grabbe, S. et al. Translating nanoparticulate-personalized cancer vaccines into clinical applications: case study with RNA-lipoplexes for the treatment of melanoma. Nanomedicine11, 2723–2734 (2016). ArticleCASPubMedGoogle Scholar
- Oberli, M. A. et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett.17, 1326–1335 (2017). ArticleCASPubMedGoogle Scholar
- Sayour, E. J. et al. Systemic activation of antigen-presenting cells via RNA-loaded nanoparticles. OncoImmunology6, e1256527 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature585, 107–112 (2020). ArticleCASPubMedGoogle Scholar
- Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature534, 396–401 (2016). ArticlePubMedGoogle Scholar
- Mendez-Gomez, H. R. et al. RNA aggregates harness the danger response for potent cancer immunotherapy. Cell187, 2521–2535.e21 (2024). ArticleCASPubMedGoogle Scholar
- Weide, B. et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J. Immunother.32, 498–507 (2009). ArticleCASPubMedGoogle Scholar
- Papachristofilou, A. et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer7, 38 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Kubler, H. et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J. Immunother. Cancer3, 26 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Cafri, G. et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Invest.130, 5976–5988 (2020). ArticleCASPubMedPubMed CentralGoogle Scholar
- Weber, J. S. et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet403, 632–644 (2024). ArticleCASPubMedGoogle Scholar
- Raimondo, T. M., Reed, K., Shi, D. N., Langer, R. & Anderson, D. G. Delivering the next generation of cancer immunotherapies with RNA. Cell186, 1535–1540 (2023). ArticleCASPubMedGoogle Scholar
- Palmer, C. D. et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results. Nat. Med.28, 1619–1629 (2022). ArticleCASPubMedGoogle Scholar
- Rappaport, A. R. et al. A shared neoantigen vaccine combined with immune checkpoint blockade for advanced metastatic solid tumors: phase 1 trial interim results. Nat. Med.30, 1013–1022 (2024). ArticleCASPubMedGoogle Scholar
- Cruz, L. J. et al. Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol.509, 143–163 (2012). ArticleCASPubMedGoogle Scholar
- Briquez, P. S. et al. Engineering targeting materials for therapeutic cancer vaccines. Front. Bioeng. Biotechnol.8, 19 (2020). ArticlePubMedPubMed CentralGoogle Scholar
- Eigentler, T. et al. A phase I dose-escalation and expansion study of intratumoral CV8102 as single-agent or in combination with anti-PD-1 antibodies in patients with advanced solid tumors. J. Clin. Oncol.38, https://doi.org/10.1200/JCO.2020.38.15_suppl.3096 (2020).
- Eigentler, T. et al. Intratumorally administered CV8102 in patients with advanced solid tumors: preliminary results from ongoing expansion part in study 008. J. Immunother. Cancer10, A818 (2022). Google Scholar
- Patel, M. R. et al. A phase I study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ, for intratumoral (iTu) injection alone and in combination with durvalumab. J. Clin. Oncol.38, 3092 (2020). ArticleGoogle Scholar
- Jimeno, A. et al. A phase 1/2, open-label, multicenter, dose escalation and efficacy study of mRNA-2416, a lipid nanoparticle encapsulated mRNA encoding human OX40L, for intratumoral injection alone or in combination with durvalumab for patients with advanced malignancies. Cancer Res.80, CT032 (2020). ArticleGoogle Scholar
- Bechter, O. et al. Abstract LB198: a first-in-human, open-label, multicenter study of intratumoral SAR441000 (mixture of cytokine encoding mRNAs), as monotherapy and in combination with cemiplimab in patients with advanced solid tumors. Phase 1/2 study of mRNA-4359 administered alone and in combination with immune checkpoint blockade in adult participants with advanced solid tumors. Cancer Res.https://doi.org/10.1158/1538-7445.AM2023-LB198 (2023). ArticleGoogle Scholar
- Barral, P. M. et al. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol. Ther.124, 219–234 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kang, D. C. et al. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene23, 1789–1800 (2004). ArticleCASPubMedGoogle Scholar
- Yoneyama, M. et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol.175, 2851–2858 (2005). ArticleCASPubMedGoogle Scholar
- Takeuchi, O. & Akira, S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol.20, 17–22 (2008). ArticleCASPubMedGoogle Scholar
- Zhang, Y. L., Guo, Y. J., Bin, L. & Sun, S. H. Hepatitis C virus single-stranded RNA induces innate immunity via Toll-like receptor 7. J. Hepatol.51, 29–38 (2009). ArticlePubMedGoogle Scholar
- Saito, T., Owen, D. M., Jiang, F., Marcotrigiano, J. & Gale, M.Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature454, 523–527 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Chattopadhyay, S. & Sen, G. C. dsRNA-activation of TLR3 and RLR signaling: gene induction-dependent and independent effects. J. Interferon Cytokine Res.34, 427–436 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Broos, K. et al. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of type I interferon. Mol. Ther. Nucleic Acids5, e326 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Napolitani, G., Rinaldi, A., Bertoni, F., Sallusto, F. & Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol.6, 769–776 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sahin, U. et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature586, 594–599 (2020). ArticleCASPubMedGoogle Scholar
- Anderson, E. J. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med.383, 2427–2438 (2020). ArticleCASPubMedGoogle Scholar
- Sittplangkoon, C. et al. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front. Immunol.13, 983000 (2022). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ramos da Silva, J. et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med.15, eabn3464 (2023). ArticleCASPubMedGoogle Scholar
- Holtkamp, S. et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood108, 4009–4017 (2006). ArticleCASPubMedGoogle Scholar
- Bonehill, A. et al. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J. Immunol.172, 6649–6657 (2004). ArticleCASPubMedGoogle Scholar
- Su, Y., Connolly, M., Marketon, A. & Heiland, T. CryJ-LAMP DNA vaccines for Japanese red edar allergy induce robust Th1-type immune responses in murine model. J. Immunol. Res.2016, 4857869 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Beck, J. D. et al. mRNA therapeutics in cancer immunotherapy. Mol. Cancer20, 69 (2021). ArticleCASPubMedPubMed CentralGoogle Scholar
- Orlandini von Niessen, A. G. et al. Improving mRNA-Based therapeutic gene delivery by expression-augmenting 3’ UTRs identified by cellular library screening. Mol. Ther.27, 824–836 (2019). ArticleCASPubMedGoogle Scholar
- Niu, D., Wu, Y. & Lian, J. Circular RNA vaccine in disease prevention and treatment. Signal. Transduct. Target. Ther.8, 341 (2023). ArticleCASPubMedPubMed CentralGoogle Scholar
- Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med.366, 2443–2454 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Mackensen, A. et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat. Med.29, 2844–2853 (2023). ArticleCASPubMedPubMed CentralGoogle Scholar
- Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol.20, 359–371 (2023). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yap, T. A. et al. A phase I/II dose escalation trial with expansion cohorts to evaluate safety and preliminary efficacy of BNT142 in patients with prospectively confirmed claudin 6-positive solid tumors J. Clin. Oncol.41, https://doi.org/10.1200/JCO.2023.41.16_suppl.TPS2669 (2023).
- Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med.383, 2603–2615 (2020). ArticleCASPubMedGoogle Scholar
- Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med.384, 403–416 (2021). ArticleCASPubMedGoogle Scholar
- Lopez, J. S. et al. A phase Ib study to evaluate RO7198457, an individualized Neoantigen Specific immunoTherapy (iNeST), in combination with atezolizumab in patients with locally advanced or metastatic solid tumors. Cancer Res.80, CT301 (2020). ArticleGoogle Scholar
- Castañón, E. et al. Intratumoral (IT) MEDI1191 + durvalumab (D): update on the first-in-human study in advanced solid tumors. Cancer Res.83, CT004 (2023). ArticleGoogle Scholar
Author information
Authors and Affiliations
- Preston A. Wells Jr. Center for Brain Tumour Therapy, University of Florida, Gainesville, FL, USA Elias J. Sayour & Duane A. Mitchell
- Lillian S. Wells Department of Neurosurgery, University of Florida, Gainesville, FL, USA Elias J. Sayour & Duane A. Mitchell
- Department of Surgery, Duke University School of Medicine, Durham, NC, USA David Boczkowski & Smita K. Nair
- Department of Neurosurgery, Duke University School of Medicine, Durham, NC, USA Smita K. Nair
- Department of Pathology, Duke University School of Medicine, Durham, NC, USA Smita K. Nair
- Duke Cancer Institute, Duke University School of Medicine, Durham, NC, USA Smita K. Nair
- Elias J. Sayour









